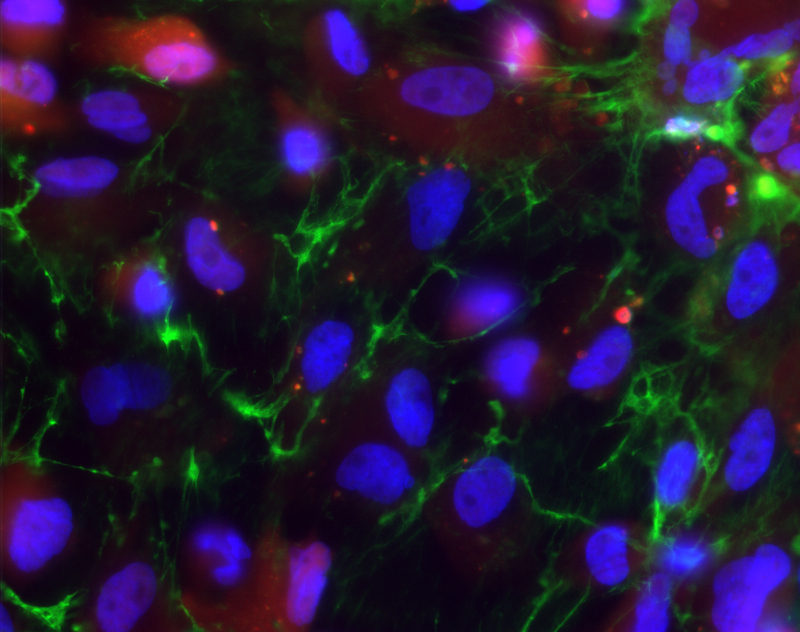
Reconstructed Cell Tray for Detection of Transplant Rejection
Graft loss continues to occur related to both acute and chronic rejection phenomenon despite the current matching and mo...
 An objective and reproducible evaluation of therapeutic candidates derived from ESCs is needed before further clinical applications can be optimized. While many ESC-derived cells appear to be attractive therapeutic options, standard and robust in vitro and in vivo tests are needed to identify those that are the most amenable to therapeutic application. We have published two original research papers in Stem Cells and Development (doi:10.1089/scd.2012.0313) and Journal of Cellular and Molecular Medicine (doi: 10.1111/jcmm.12002), which defined our protocol to derive progenitor cells from embryonic stem cells. We have developed a procedure how to denude the endothelium of baboon blood vessels, which will be used for our model to evaluate the therapeutic activity. In the initial studies using two baboons, we used a procedure modified based on previous reports in order to be applicable in baboons. The procedure will follow current standard operating procedure (SOP) that is currently under development and validation by a team consisting of investigators involved in this project.
An objective and reproducible evaluation of therapeutic candidates derived from ESCs is needed before further clinical applications can be optimized. While many ESC-derived cells appear to be attractive therapeutic options, standard and robust in vitro and in vivo tests are needed to identify those that are the most amenable to therapeutic application. We have published two original research papers in Stem Cells and Development (doi:10.1089/scd.2012.0313) and Journal of Cellular and Molecular Medicine (doi: 10.1111/jcmm.12002), which defined our protocol to derive progenitor cells from embryonic stem cells. We have developed a procedure how to denude the endothelium of baboon blood vessels, which will be used for our model to evaluate the therapeutic activity. In the initial studies using two baboons, we used a procedure modified based on previous reports in order to be applicable in baboons. The procedure will follow current standard operating procedure (SOP) that is currently under development and validation by a team consisting of investigators involved in this project. We propose to generate a vascular replacement based on complete reconstitution by using three vascular components (endothelial cells, smooth muscle cells, and pericytes) together with biodegradable scaffolds with topological characteristics similar to native vasculature. We will explore the processes needed to construct the conduit with defined vascular components and we will determine the conditions under which these components can resume function under experimental manipulation. If we are successful, we can produce a novel biologic product that yields a living vascular replacement with responsiveness to environmental stimuli, with potential to grow according to local regulation, and with sufficient mechanical strength and readiness for suturing; these replacements will be nonthrombogenic and self-repairing. If adequate cell sources are located, our proposed process will reduce both the time and the cost of manufacturing. Following GMP regulations, we will be able to make consistently useable products that can be stored as off-the-shelf medical biologicals and used in emergencies.
We propose to generate a vascular replacement based on complete reconstitution by using three vascular components (endothelial cells, smooth muscle cells, and pericytes) together with biodegradable scaffolds with topological characteristics similar to native vasculature. We will explore the processes needed to construct the conduit with defined vascular components and we will determine the conditions under which these components can resume function under experimental manipulation. If we are successful, we can produce a novel biologic product that yields a living vascular replacement with responsiveness to environmental stimuli, with potential to grow according to local regulation, and with sufficient mechanical strength and readiness for suturing; these replacements will be nonthrombogenic and self-repairing. If adequate cell sources are located, our proposed process will reduce both the time and the cost of manufacturing. Following GMP regulations, we will be able to make consistently useable products that can be stored as off-the-shelf medical biologicals and used in emergencies. In this project, we will refine protocols to enable the production of sufficient amount EPCs that are compliant with applicable current Good Manufacturing Practice (cGMP), characterize their composition and biological features, and evaluate robustly their therapeutic potencies for endothelial reconstitution in vivo. Eventually, we will formulate them as clinical-stage-appropriate products for further research and development. Deriving EPCs from ESCs and testing their efficacy in the same species, especially in baboons, will fundamentally impact embryonic stem cell therapy because 50% of clinical trails of cell therapeutics are conducted without an understanding of their mode of action, which has resulted in a significant economic loss. An objective and reproducible evaluation of therapeutic biologics is needed before further clinical application can be optimized. While many ESC-derived cells appear to be attractive options, standard and robust in vitro and in vivo tests in a suitable animal model can identify those that are the most amenable to therapeutic application.
In this project, we will refine protocols to enable the production of sufficient amount EPCs that are compliant with applicable current Good Manufacturing Practice (cGMP), characterize their composition and biological features, and evaluate robustly their therapeutic potencies for endothelial reconstitution in vivo. Eventually, we will formulate them as clinical-stage-appropriate products for further research and development. Deriving EPCs from ESCs and testing their efficacy in the same species, especially in baboons, will fundamentally impact embryonic stem cell therapy because 50% of clinical trails of cell therapeutics are conducted without an understanding of their mode of action, which has resulted in a significant economic loss. An objective and reproducible evaluation of therapeutic biologics is needed before further clinical application can be optimized. While many ESC-derived cells appear to be attractive options, standard and robust in vitro and in vivo tests in a suitable animal model can identify those that are the most amenable to therapeutic application. Traditional tissue engineering methods have led to the construction of blood vessel grafts by seeding biodegradable scaffolds with cells isolated from adult tissues. As the new tissue grows, the scaffold degrades, leaving a purely biological graft. Pre-clinical studies have shown that the primary mode of failure in these grafts is stenosis (narrowing of the internal opening of the vessel). It seems that even though the cells are supplied with the necessary biological and physical stimuli to coax them towards vascular formation ex vivo, these conduits lack the multitude of cues present in the in vivo environment to prevent graft failure. Therefore, a better technology is needed to enable rational design of durable engineered vascular tissues to be used in the clinical setting.
Traditional tissue engineering methods have led to the construction of blood vessel grafts by seeding biodegradable scaffolds with cells isolated from adult tissues. As the new tissue grows, the scaffold degrades, leaving a purely biological graft. Pre-clinical studies have shown that the primary mode of failure in these grafts is stenosis (narrowing of the internal opening of the vessel). It seems that even though the cells are supplied with the necessary biological and physical stimuli to coax them towards vascular formation ex vivo, these conduits lack the multitude of cues present in the in vivo environment to prevent graft failure. Therefore, a better technology is needed to enable rational design of durable engineered vascular tissues to be used in the clinical setting.