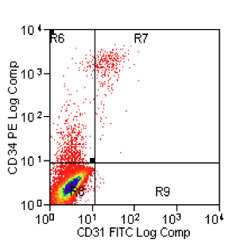
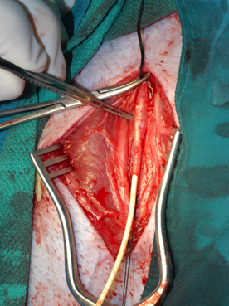
An objective and reproducible evaluation of therapeutic candidates derived from ESCs is needed before further clinical applications can be optimized. While many ESC-derived cells appear to be attractive therapeutic options, standard and robust in vitro and in vivo tests are needed to identify those that are the most amenable to therapeutic application. We have published two original research papers in Stem Cells and Development (doi:10.1089/scd.2012.0313) and Journal of Cellular and Molecular Medicine (doi: 10.1111/jcmm.12002), which defined our protocol to derive progenitor cells from embryonic stem cells. We have developed a procedure how to denude the endothelium of baboon blood vessels, which will be used for our model to evaluate the therapeutic activity. In the initial studies using two baboons, we used a procedure modified based on previous reports in order to be applicable in baboons. The procedure will follow current standard operating procedure (SOP) that is currently under development and validation by a team consisting of investigators involved in this project.